Admission CTAs
Innovation Pharmaceuticals and George Mason University Release Laboratory Testing Results Demonstrating Brilacidin’s COVID-19 Treatment Potential
The preprint article, being submitted for peer-review publication, details research conducted at George Mason’s Regional Biocontainment Laboratory:
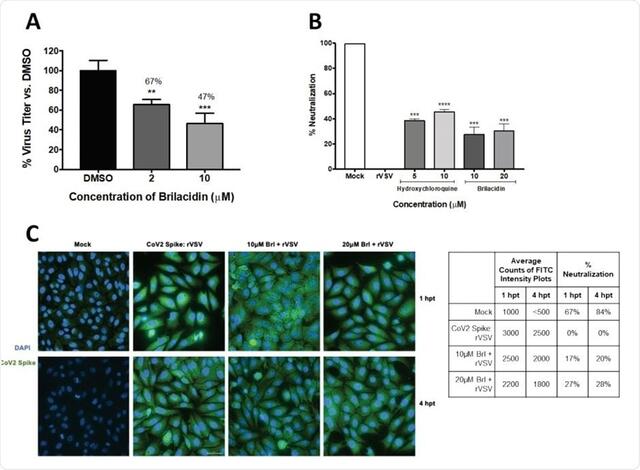
- Brilacidin shown in vitro to potently inhibit SARS-CoV-2, the novel coronavirus causing COVID-19.
- In a human lung cell line infected by SARS-CoV-2, Brilacidin achieved a high Selectivity Index of 426.
- The primary mechanism of action of Brilacidin appears to disrupt viral integrity and block viral entry.
- Brilacidin and FDA-approved remdesivir exhibit excellent synergistic activity in vitro against SARS-CoV-2.
October 30, 2020
Innovation Pharmaceuticals, of Wakefield, MA, a clinical stage biopharmaceutical company, and George Mason University’s National Center for Biodefense and Infectious Diseases (NCBID), jointly announced completion of extensive laboratory testing supporting anti-SARS-CoV-2 activity of Brilacidin, a defensin-mimetic drug candidate, which is being developed as a potential COVID-19 treatment.
“We thank the scientists who have conducted an impressive amount of antiviral research on Brilacidin, which strongly supports its treatment potential in the fight against COVID-19,” commented Leo Ehrlich, Chief Executive Officer at Innovation Pharmaceuticals. “It is timely to have Brilacidin anchored in such sound science, as we anticipate commencing soon a clinical trial of Brilacidin for treatment of COVID-19. We look forward to continuing our antiviral research collaborations, while Brilacidin advances into clinical testing against COVID-19, where we believe Brilacidin can have a beneficial patient impact.”
“During the global COVID-19 pandemic, we selectively formed new external industry partnerships, including this opportunity to research a drug candidate like Brilacidin,” said Aarthi Narayanan, Associate Professor of Systems Biology in Mason’s College of Science. “In testing at GMU’s BSL-3 lab, we showed that Brilacidin potently inhibits SARS-CoV-2 in vitro against the live virus. Beyond exhibiting treatment potential for those already infected by COVID-19, Brilacidin’s ability to disrupt viral integrity and block viral entry indicates it has the added potential to prevent infection, upon appropriate formulation, as a prophylactic. I look forward to working with Innovation to investigate further Brilacidin’s antiviral properties,” Narayanan added.
“Brilacidin’s demonstration of potent antiviral activity against SARS-CoV-2 is important, particularly given its apparent ability to impact the virus directly,” said William F. DeGrado, PhD, Professor in the Department of Pharmaceutical Chemistry at University of California San Francisco and Scientific Advisor to Innovation Pharmaceuticals. “This antiviral mechanism of action suggests Brilacidin might be able to inhibit other viruses and be less prone to resistance developing due to viral mutations—a weak spot for other antivirals, antibody-based treatments and vaccines. I am extremely hopeful Brilacidin will emerge as an effective COVID-19 treatment given the ongoing worldwide need for COVID-19 therapeutics.”
Brilacidin and COVID-19
Brilacidin is one of the few drugs targeting COVID-19 that has been tested in human trials (a total of 8) for other clinical indications, providing established safety and efficacy data on over 460 subjects, thereby potentially enabling it to rapidly help address the novel coronavirus crisis. Laboratory testing at independent laboratories supports Brilacidin’s antiviral ability to safely and potently inhibit SARS-CoV-2. In a human lung cell line, Brilacidin achieved a Selectivity Index of 426. A molecular screening study of 11,552 compounds also supports Brilacidin as a promising novel coronavirus treatment. Additional pre-clinical and clinical data support Brilacidin’s inhibition of IL-6, IL-1β, TNF-α and other pro-inflammatory cytokines and chemokines, which have been identified as central drivers in the worsening prognoses of hospitalized COVID-19 patients. Brilacidin’s robust antimicrobial properties might also help to fight secondary bacterial infections, which can co-present in up to 20 percent of COVID-19 patients. Collectively, these data support Brilacidin as a unique 3 in 1 combination—antiviral, immuno/anti-inflammatory, and antimicrobial—COVID-19 therapeutic candidate.
About Innovation Pharmaceuticals
Innovation Pharmaceuticals Inc. (IPIX) is a clinical stage biopharmaceutical company developing a world-class portfolio of innovative therapies addressing multiple areas of unmet medical need, including inflammatory diseases, cancer, infectious diseases, and dermatologic diseases. Brilacidin, a versatile compound with broad therapeutic potential, is in a new chemical class called defensin-mimetics. A Phase 2 trial of Brilacidin as an oral rinse for the prevention of Severe Oral Mucositis (SOM) in patients with Head and Neck Cancer, met its primary and secondary endpoints, including reducing the incidence of SOM. The Company plans to advance Brilacidin oral rinse into Phase 3 development, subject to available financial resources. Positive results were also observed in a Phase 2 Proof-of-Concept trial treating patients locally with Brilacidin for Ulcerative Proctitis/Ulcerative Proctosigmoiditis (UP/UPS). Brilacidin for UP/UPS was licensed to Alfasigma S.p.A. in July 2019. A Phase 2b trial of Brilacidin showed a single intravenous dose of the drug delivered comparable outcomes to a seven-day dosing regimen of the FDA-approved blockbuster daptomycin in treating Acute Bacterial Skin and Skin Structure Infection. Brilacidin, based on promising in vitro antiviral activity against SARS-CoV-2, is being evaluated as a potential treatment for COVID-19. Kevetrin is a novel anti-cancer drug shown to modulate p53, often referred to as the “Guardian Angel Gene” due to its crucial role in controlling cell mutations and has successfully completed a Phase 2 trial in Ovarian Cancer. More information is available on the Company website at www.IPharmInc.com.
About the Biomedical Research Laboratory (BRL) at George Mason University
George Mason University Biomedical Research Laboratory (BRL) is one of thirteen Regional Biocontainment Laboratories constructed with funding support from the National Institute of Allergy and Infectious Diseases/National Institutes of Health (NIAID/NIH). The BRL is a state-of-the-art laboratory with biosafety level 3 and aerosolization capabilities where scientists perform pioneering research of infectious diseases, both emerging and potential bio threat agents. Learn more at ncbid.gmu.edu.
Forward-Looking Statements: This post contains forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 including statements concerning future drug development plans, statements regarding the antiviral capabilities and therapeutic potential of Brilacidin and its impact on SARS-CoV-2 (COVID-19) and other coronaviruses, as well as obtaining government regulatory approvals to commence clinical testing. Other statements regarding future product developments, and markets, including with respect to specific indications, and any other statements which are other than statements of historical fact. These statements involve risks but not limited to risks related to conducting pre-clinical studies and clinical trials and seeking IND regulatory approval for Brilacidin and Kevetrin; that prior test results may not be replicated in future studies and trials, uncertainties and assumptions that could cause the Company’s actual results and experience to differ materially from anticipated results and expectations expressed in these forward-looking statements. The Company has in some cases identified forward-looking statements by using words such as “anticipates,” “believes,” “hopes,” “estimates,” “looks,” “expects,” “plans,” “intends,” “goal,” “potential,” “may,” “suggest,” and similar expressions. Among other factors that could cause actual results to differ materially from those expressed in forward-looking statements are the Company’s need for, and the availability of, substantial capital in the future to fund its operations and research and development; including the amount and timing of the sale of shares of common stock under securities purchase agreements; the fact that the Company’s licensee(s) may not successfully complete pre-clinical or clinical testing and the Company will not receive milestone payments, or the fact that the Company’s compounds may not successfully complete pre-clinical or clinical testing, or be granted regulatory approval to be sold and marketed in the United States or elsewhere. A more complete description of these risk factors is included in the Company’s filings with the Securities and Exchange Commission. You should not place undue reliance on any forward-looking statements. The Company undertakes no obligation to release publicly the results of any revisions to any such forward-looking statements that may be made to reflect events or circumstances after the date of this press release or to reflect the occurrence of unanticipated events, except as required by applicable law or regulation.